Characteristics of graphite
LUBRICATIVE PERFORMANCE
Graphite provides superb lubrication and its properties undergo almost no change even at high temperatures or under heavy loads. Thanks to its perfect suitability as a maintenance-free method of lubrication, the use of graphite is constantly expanding.
ELECTRIC CONDUCTIVITY
Graphite’s resistivity is 2~4×10-2 Ωcm, which is a bit inferior to that of such metals as copper, silver and gold, but because graphite is less likely to be oxidized than copper and is cheaper than silver or gold, it is more widely used.
THERMAL CONDUCTIVITY
Heat has a transfer mechanism like electricity, so graphite offers excellent heat conductivity. In fact, it is added often to refractory, rubber, resin, and the like for the purpose of improving heat conductivity.
HEAT RESISTANCE
Graphite is subject to oxidation wear at temperatures exceeding 500℃ in an oxidizing atmosphere bun in a non-oxidizing atmosphere it remains stable up to 3,500℃ and offers excellent thermal resistance. It is commonly used in refractory materials, including magnesia carbon bricks for the iron & steel industry.
RESISTANCE TO CHEMICALS
Graphite possesses the covalent crystalline structure of carbon, which makes it extremely stable. It is stable in the presence of both acid and alkaline substances, and it has outstanding resistance to chemicals.
Classification of graphite
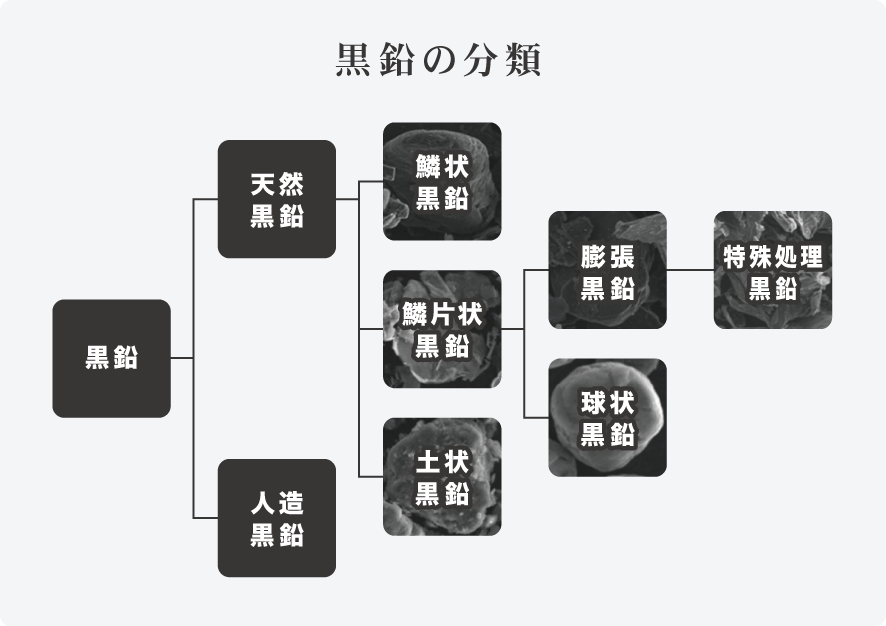
Flake Graphite
The most graphitized graphite with a spathic crystalline appearance, mainly produced in China, Brazil, Ukraine, etc.
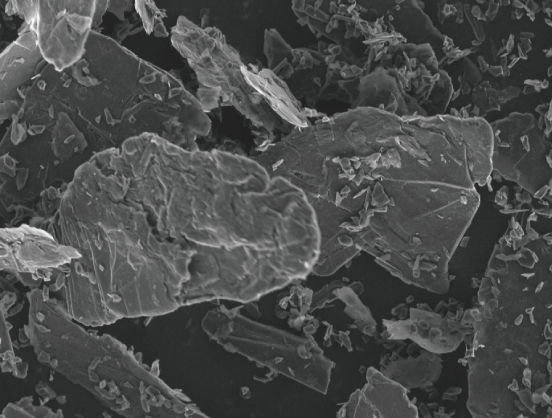
Vein Graphite
Veined massive graphite, produced in Sri Lanka
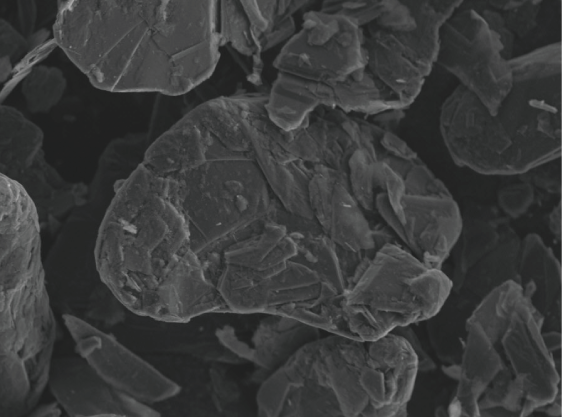
Amorphous Graphite
Amorphous graphite with an earthy or clod-like appearance, mainly produced in China, etc.
Synthetic Graphite
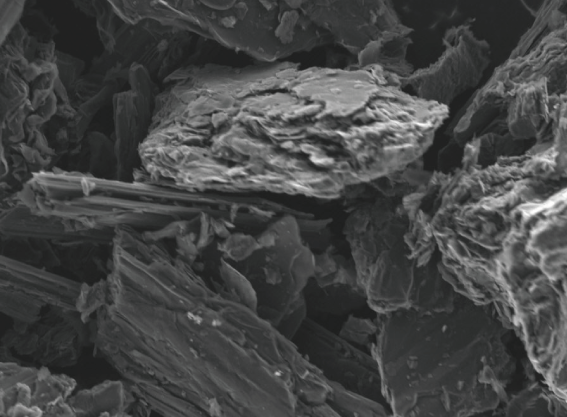
Crushed and classified synthetic graphite electrode shavings
Expandable Graphite
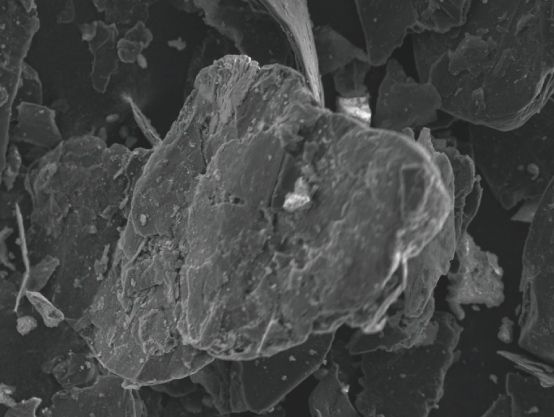
Expandable graphite is made by treating flake graphite chemically
Special Treated Graphite

Crushed expanded graphite formed by heat treatment with high bulk specific gravity
Spherical Graphite
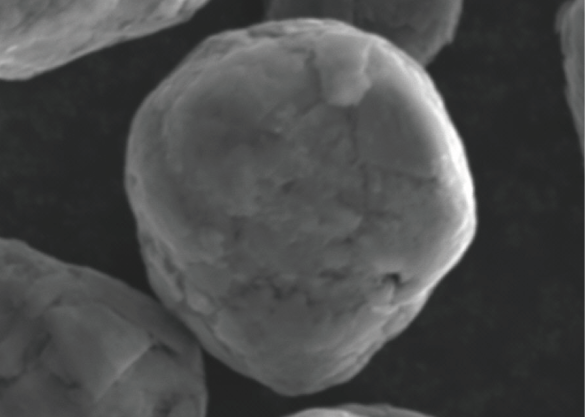
Spherically-shaped flake graphite. Its powders alone are spherical grains, so they exhibit the properties of graphite in the vertical direction.