Utilization of Graphite in Resin

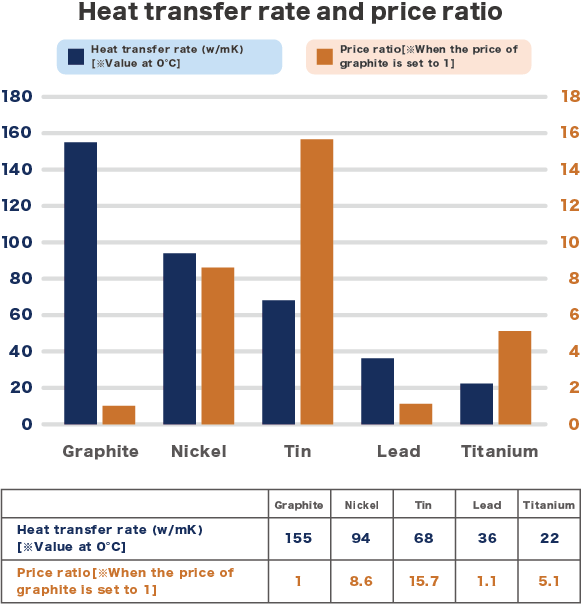
Graphite has good electrical conductivity, thermal conductivity, heat resistance, chemical resistance, and lubricity. Graphite with these properties is used in a variety of applications, including automotive parts, electronic devices, and OA devices. In recent years, electronic devices have become smaller and more functional, and the need for heat dissipation has become even greater. Therefore, the addition of graphite to resin to improve heat dissipation has attracted attention, and there are now many cases where heat dissipation has been improved. At the same time, graphite has gained attention for its lower specific gravity and lower cost compared to metals, and thus there is a growing trend to use graphite to replace other materials.
Enhance the technology of thermal conductivity through spheronization of graphite powder
Physical properties of graphite powder and its properties
Graphite is one of the allotropes of carbon (C) with a metallic luster(carbon allotropes include diamond, coal, carbon black, coke, etc). However, its shape and properties differ from other carbon allotropes due to the difference in crystal structure.
The crystal structure of graphite is a layered structure in which hexagonal network planes of carbon atoms are stacked in a regular manner. The bonds between the carbon atoms are composed of two types of bonds: covalent bonds and van der Waals forces. The bonds inside the layered planes (horizontal axis) are the planes where carbon atoms are bonded by covalent bonds (base plane) and have strong bonding forces, while the bonds between the layers (vertical axis) are weak electronic bonds due to van der Waals forces. In addition, graphite has high electrical conductivity because electrons move like free electrons in the hexagonal network planes of the crystal structure, but it also has high thermal conductivity like metals because the free electrons act as a medium for heat. Other properties of graphite include lubricity, heat resistance, durability, and chemical resistance.
In other words, graphite is a light material that possesses excellent heat resistance and durability, which are the most characteristic properties of ceramics, and high electrical and thermal conductivity, which are characteristic properties of metals. Because of these characteristic properties, graphite is used in a variety of applications.
Current status of high thermal conductivity technology for plastics
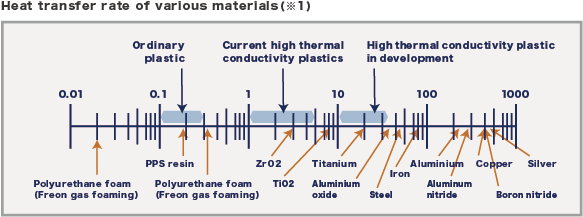
The thermal conductivity of plastics is generally very low compared to metals and ceramics (picture 1).
For this reason, plastic has been used in various fields as a compounding material for gases and as a heat insulator. Since about 20 years ago, however, it has become desirable to increase the thermal conductivity of plastics with excellent moldability in order to improve heat dissipation, especially in the electronics field. Nevertheless, there is a limit to the high thermal conductivity of the plastic itself. Therefore, plastic has been mixed with highly thermally conductive fillers to increase the thermal conductivity.
As fillers, aluminum powder, copper powder, and graphite powder are used for the conductive type, and boron titride, aluminum titride, and alumina are used for the electrically insulating type. However, unlike electrical conductivity, the thermal conductivity of composite plastics does not exhibit percolation phenomenon, and does not increase much in the low-filling region, unless high filling is up to 50-60 volume% capacity. However, high filler filling has the problem of causing an extreme decrease in moldability. Therefore, the thermal conductivity of the high thermal conductivity plastics is often up to 5 W/m-K. Graphite powder, a filler with relatively low specific gravity, is expected to be used to make the best use of the lightweight property of plastics. However, since graphite powder is generally flat, it tends to be oriented during the forming process, and the thermal conductivity in the thickness direction of the composite film cannot be improved. So, we have investigated the use of spherical graphite powder and found that the problem can be solved to some extent.
Next, the current status of the use of graphite powder in high thermal conductivity plastics is presented, followed by an explanation of the effects and possibilities of the use of spherical graphite powder.
Use of graphite powder for high thermal conductivity
Research has been carried out on the thermal conductivity of plastics filled with graphite powder, as well as copper and alumina powders.
We have also investigated the effect of different dispersion methods on polyethylene filled with graphite powder. The dispersion of the particles is highly variable depending on the preparation method and the property of filler particles and polymer. Even in the case of macroscopically non-anisotropic and randomly dispersed particles, the particles may have a variety of short-range orders and show different dispersion states depending on the ways of making samples and the property of the continuous medium. Therefore, it is known that the percolation concentration in various particle-dispersed composite systems can vary from 5 to 30 volume% based on electrical conductivity measurements, even though the theoretical calculation shows that the percolation concentration of the dispersed particles is around 30 volume%. This difference in dispersion also influences the effective thermal conductivity. In addition, the usual theoretical equation assumes the thermal conductivity of the composite is "homogeneous" or "fully dispersed" and does not provide an influence factor for the state of dispersion.
Therefore, there are many cases where the thermal conductivity differs greatly in spite of a similar composite system, e.g. the thermal conductivity of a graphite powder (18volume%) composite polyethylene of the same composition was approximately doubled when the dispersion state changed and the percolation concentration was reduced to 1/3 of the original value. When these electrical conductivities were examined, the CVF values (concentration of the filler particles at which the second filling region begins :the inflection point of the S-curve in the relationship between the logarithmic value of the electrical conductivity vs. the volume fraction of the filler particles) also varied greatly. The value was smaller when the thermal conductivity was larger. This suggests that the thermal conductivity is larger in the systems where continuums of filler particles are easily formed.
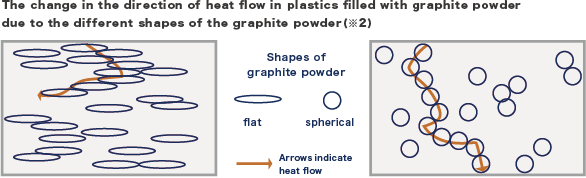
We also found that the effect of filler shape can be explained by our predictive equation, as well as the effect of dispersion state. Graphite particles are generally flat, aligned in the flow direction and heat is easily transferred in the direction of the film surface, making them useful as heat spreaders, and they are still commonly used today. However, due to their low thermal conductivity in the thickness direction of the composite film, they are not suitable for use in applications where high thermal conductivity in the thickness direction of the composite film is expected, such as adhesives and heat conductive sheets, which take advantage of one of the characteristics of plastics-flexibility.
This is one of the most significant areas of use in combination with metals, such as heat sinks.
Spherical graphite powders have therefore been developed, which are expected to reduce these effects.
Here, we mixed spherical graphite powder with polypropylene and compared the thermal conductivity with that of polypropylene mixed with ordinary flat graphite powder. As shown in picture 2, the heat flow tends to go towards the surface direction in the case of mixing flat graphite powder, but we think that the heat flow also goes towards the thickness direction effectively in the case of mixing spherical graphite powder. When the thermal conductivity in the thickness direction of the film was measured, the thermal conductivity of the film mixed spherical graphite powder showed greater than that of the film mixed flat graphite powder (picture 3). The difference was greater at higher filling rates, especially at 80 wt%, the thermal conductivity with the addition of spherical graphite was 40-50% greater than that with the addition of flat graphite.
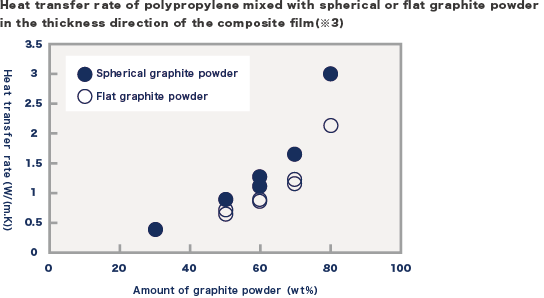
We found that the anisotropy is greatly reduced when spherical graphite powder is used, which can minimise the disadvantages of graphite powder so far. Therefore, it is expected that the use of spherical graphite powder can improve the thermal conductivity in the thickness direction of the composite film and expand the fields of application of high thermal conductivity polymer materials by taking advantage of the light weight and other characteristics of graphite powder.
Future prospects for high thermal conductivity technology using carbon-based fillers
Carbon-based additives such as graphite powder have high thermal conductivity like metals, but their specific gravity is relatively low, so when they are combined with plastics, lightweight, high thermal conductivity plastics can be obtained. It is expected to be used in recently growing fields such as mobile automobiles, mobile phones and notebook computers. It is also expected to be used as a heat-dissipating material for fuel cells, since the metal ions do not dissolve in acidic solutions like metal powders do.
Carbon materials range from those with a thermal conductivity as high as aluminum to those with a thermal conductivity more than twice that of copper, and can be selected according to the purpose of use. Therefore, various carbon materials, including spherical graphite powder, are expected to be widely used as highly thermally conductive fillers in fields where various heat dissipation properties are required.
参考文献
1)D.E.Kline,Hansen:“Thermal Characterization Technique”,P.E.Slade,Jr.,L.T.Jenkins ed.,Mercel Dekker,Inc.,1970
2)D.M.Bigg:Polym.Eng.Sci.,19,1188(1979)
3)山田悦郎:熱物性,3,78(1989)
4)金成克彦:小沢丈夫,熱物性,3,106(1989)
5)Y.Agari,A.Ueda,S.Nagai:J.Appl.Polym.Sci.,49,1625(1993)
6)荒木信幸ら:“レーザーフラッシュ法等の非定常法による熱伝導率/熱拡散率評価技術調査報告書”,(社)日本機械工業連合会,(株)超高温材料研究所,(1997)
7)上利泰幸,紙谷畑恒雄:日系エレクトロニクス12月16日号,127(2002)
8)上利泰幸,材料の科学と工学,42,308(2005)
9)上利泰幸,材料の科学と工学,42,308(2005)
10)Y.Agari,A.Ueda,S.Nagai:J.Appl.Polym.Sci.,43,1117(1991)